Menu
Tumor medication
Common Medications
{{ variable.name }}
In April 2019, Amgen/UCB announced that the FDA approved its osteoporosis treatment drug Evenity (romosozumab) for the treatment of osteoporosis in postmenopausal women with a high risk of fractures. Romosozumab-aqqg is a humanized IgG2 monoclonal antibody targeting sclerostin and the world's first monoclonal antibody targeting sclerostin.
[English name]: Evenity (romosozumab-aqqg)
[R&D company]: Amgen
[Indications]: Treatment of postmenopausal women with high osteoporosis after fracture
[Model and specification]: Injection, specification is 105mg/1.17ML.
[Evenity (romosozumab-aqqg) indications]
Treatment of postmenopausal women with high osteoporosis after fracture
Evenity is indicated for the treatment of osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple fracture risk factors; or in patients who have failed or are intolerant to other available osteoporosis treatments.
Limitations of use:
After 12 months of treatment, the anabolic effects of Evenity diminish. Therefore, the duration of Evenity use should be limited to a 12-month dose. If osteoporosis treatment is still required, continued treatment with an antiresorptive agent should be considered [see Dosage and Administration and Clinical Studies].
[Evenity (romosozumab-aqqg) dosage and administration]
1. Important dosage and administration instructions
1. Two separate syringes (and two separate subcutaneous injections) are required to administer a total dose of 210 mg Evenity. Fill two 105 mg/1.17 mL prefilled syringes one after the other.
2.Evenity should be administered by a healthcare provider.
2. Recommended dosage
1. The recommended dosage of Evenity is 210 mg subcutaneously injected into the abdomen, thigh or upper arm. EVENITY once a month.
2. The treatment duration of Evenity is 12 months of dosage.
3. During treatment with Evenity, patients should fully supplement calcium and vitamin D [see Warnings and Precautions and Clinical Studies].
4. If an Evenity dose is missed, it can be administered immediately after rescheduling. Thereafter, Evenity can be scheduled monthly starting from the date of the last dose.
[Evenity (romosozumab-aqqg) Precautions]
1. Major adverse cardiac events (MACE): Monitor for symptoms of myocardial infarction. If symptoms occur, stroke and seek prompt medical attention. (5.1)
2. Hypersensitivity: allergic reactions, including angioedema, erythema multiforme, dermatitis, rash and urticaria. Discontinue EVENITY if a clinically significant allergic reaction occurs.
3. Hypocalcemia: Fully supplement calcium and vitamin D during treatment with EVENITY.
4. Osteonecrosis of the mandible: Monitor symptoms. Consider discontinuing treatment based on welfare risk assessment.
5. Atypical Femur Fracture: Evaluate new or unusual pain in the thigh, hip, or groin to rule out incomplete femoral fracture.
[Evenity (romosozumab-aqqg) adverse reactions]
The most common adverse reactions (≥5%) reported by Evenity in clinical trials were joint pain and headache.
[Evenity (romosozumab-aqqg) dosage form]
Injection: 105mg/1.17mL clear to milky white, colorless to light yellow solution in disposable prefilled syringe.
A full dose of Evenity requires two disposable, prefilled syringes.
Evenity (romosozumab-aqqg) injection is a clear, opalescent, colorless to yellowish solution for subcutaneous injection, supplied in a disposable prefilled syringe.
Each single-use prefilled syringe contains 105 mg of Evenity and delivers a volume of 1.17 ml. To provide a complete dose, two 105 mg/1.17 mL EVENITY prefilled syringes are injected one after the other for a total dose of 210 mg.
NDC 55513-880-02: Carton of two 105 mg/1.17 mL disposable prefilled syringes.
Prefilled syringes are not made from natural rubber latex.
[Evenity (romosozumab-aqqg) preparation and administration instructions]
Step 1. Before administration
1. Take out two syringes from the carton.
2. Visually inspect Evenity particles and discoloration before administration. Evenity is a clear to milky white, colorless to pale yellow solution. Do not use if solution is cloudy or discolored or contains particles.
3. Please do not use the syringe if
*Any part may break or break
*Gray needle cap is missing or not securely attached
*The expiry date printed on the label has passed
4. Always hold the prefilled syringe by the syringe barrel and remove the syringe from the tray. See Figure A.
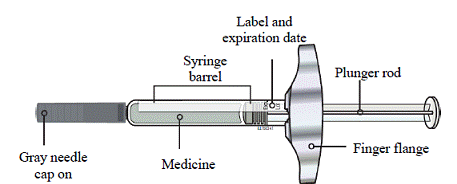
*Do not hold onto the plunger rod.
*Do not grab the gray needle cap.
*Do not remove the gray needle cap until you are ready to inject.
5. Let Evenity sit at room temperature for at least 30 minutes before injection. Do not heat in any other way.
Step 2: Select injection site and prepare syringe
Prepare and clean both injection sites, one for each injection. See Figure B.
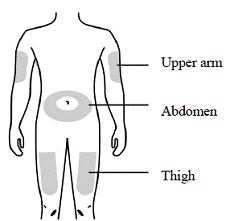
Recommended subcutaneous injection sites include:
1. Thigh
2. Abdomen, except the two-inch area around the belly button
3. External upper arm area
Clean the injection site with an alcohol wipe. Let skin dry.
Choose a different site for each injection. If you want to use the same injection site, make sure it is not the same injection site you used for the previous injection.
Do not inject into areas of soft, bruised, red, or hard skin. Avoid injecting into areas with scars or stretch marks.
Select the first syringe. When you are ready to inject, pull the gray needle cover directly away from your body. See Figure C.
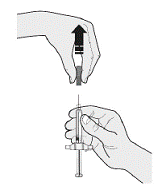
Do not put the gray needle cap back on the syringe.
Step 3: Inject Evenity
Insert the needle and inject all the fluid under the skin. Do not enter muscles or blood vessels. See Figure d
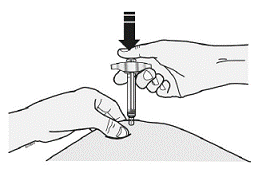
When finished, gently remove the syringe from the skin.
Step 4: Syringe and Needle Cap Disposal
Immediately place the syringe and needle cap in the nearest sharps container.
Important: Repeat all steps using a second syringe to inject the full dose.
[Evenity (romosozumab-aqqg) Storage and Handling]
1. Refrigerate Evenity in the original carton at 2°C to 8°C (36°F to 46°F), protected from light. Do not freeze. Stop shaking.
2. If removed from the refrigerator, Evenity can be stored in the original carton at room temperature up to 25°C (77°F) and must be used within 30 days. If not used within 30 days, discard Evenity.
3. Do not expose Evenity to temperatures above 25°C (77°F).