Menu
Tumor medication
Common Medications
{{ variable.name }}
[Drug name]
Common name: Apixaban Tablets
English name: Apixaban Tablets
Trade name: Eliquis
[Ingredients]
Active ingredient of this product: Apixaban
[Characteristics]
This product is a yellow film-coated tablet that appears white to off-white after the coating is removed.
[Indications]
For adult patients undergoing elective hip or knee replacement to prevent venous thromboembolic events (VTE).
[Specifications]
5mg
[Usage and Dosage]
The recommended dosage of this product is 2.5mg each time, taken orally twice a day with water, and will not be affected by meals. The first medication should be taken between 12 and 24 hours after surgery. When deciding when to take medication during this time window, physicians need to consider both the potential benefit of early anticoagulation to prevent VTE and the risk of postoperative bleeding.
For patients undergoing hip replacement: the recommended course of treatment is 32 to 38 days
For patients undergoing knee replacement: the recommended course of treatment is 10 to 14 days.
If a dose is missed, the patient should take this product immediately and continue to take it twice daily. When switching from injectable anticoagulants to treatment with this product, treatment can start from the next administration time point (and vice versa). See [Drug Interactions].
[Adverse reactions]
The safety of apixaban was evaluated in one phase II clinical trial and three phase III clinical trials. In these trials, a total of 5924 patients who underwent major lower limb orthopedic surgery (elective hip replacement or knee replacement) took apixaban 2.5 mg three times a day for up to 38 days of treatment.
A total of 11% of patients treated with apixaban 2.5 mg twice daily experienced adverse reactions. As with other anticoagulants, bleeding may occur during apixaban treatment when associated risk factors are present, such as organ damage that predisposes to bleeding. Common adverse reactions include anemia, bleeding, bruising and nausea. Adverse effects should be explained in the context of the procedure.
In Table 1 below, the adverse reactions in the above-mentioned Phase II and Phase III clinical trials are listed according to system organ classification (MedDRA) and frequency of occurrence.
Table 1: Adverse reactions occurring during treatment in patients undergoing elective hip or knee replacement surgery
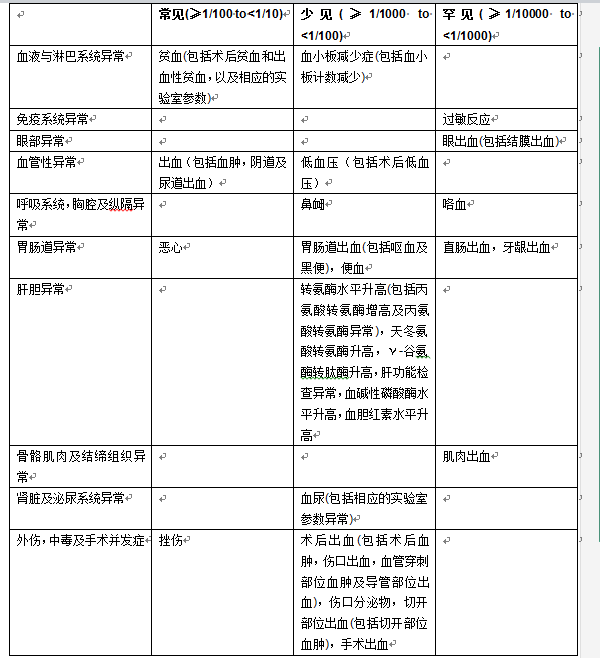
[Contraindications]
Allergic to the active ingredients or any excipients in the tablets; clinically significant active bleeding; accompanied by Liver diseases with coagulation abnormalities and clinically relevant bleeding risk (refer to [Pharmacokinetics])
[Precautions]
Bleeding risk
As with other anticoagulants, patients taking apixaban should be closely monitored for signs of bleeding. Apixaban should be used with caution in patients with the following bleeding risks: congenital or acquired bleeding disorders; active gastrointestinal ulcerative disease; bacterial endocarditis; thrombocytopenia; platelet function abnormalities; a history of hemorrhagic stroke; uncontrolled severe hypertension; and recent brain, spine, or ophthalmic surgery. If severe bleeding occurs, apixaban should be discontinued (see Overdose).
Renal Impairment
No dose adjustment is required in patients with mild or moderate renal impairment (see [Pharmacokinetics]).
Limited clinical data in patients with severe renal impairment (creatinine clearance 15-29 ml/min) indicate that apixaban plasma concentrations are elevated in this patient population, and apixaban should be used with caution alone or in combination with acetylsalicylic acid in these patients due to the potential for increased bleeding risk (see Pharmacokinetics).
Since there are no clinical data on patients with creatinine clearance <15ml/min or dialysis patients, apixaban is not recommended for these patients (see [Pharmacokinetics]).
Elderly patients
Clinical experience with the combination of apixaban and acetylsalicylic acid in elderly patients is limited. Elderly patients should be cautious when taking these two drugs together due to the possible increased risk of bleeding.
Liver Impairment
Apixaban is contraindicated in patients with liver disease associated with coagulation abnormalities and clinically relevant risk of bleeding (see Contraindications).
Apixaban is not recommended for patients with severe hepatic impairment (see [Pharmacokinetics]).
Apixaban should be taken with caution in patients with mild to moderate hepatic impairment (Child Pugh class A or B) (see [Pharmacokinetics]).
Because patients with elevated liver enzymes ALT/AST>2*ULN or elevated total bilirubin ≥1.5*ULN were not selected for clinical trials, apixaban should be used with caution in these groups (see [Pharmacokinetics]). ALT should be routinely tested before surgery.
Interactions with cytochrome P4503A4 (CYP3A4) and P-glycoprotein (P-gp) inhibitors:
Apixaban is not recommended for patients taking systemic therapy with strong CYP3A4 and P-gp inhibitors: such inhibitors include azole antifungals (such as ketoconazole, itraconazole, voriconazole and posaconazole) and HIV protease inhibitors (such as ritonavir). These drugs can increase the average ACU of apixaban by 2-fold (see [Drug Interactions]). If other factors that increase apixaban exposure (such as severe renal impairment) are present, the average ACU of apixaban will increase even more.
Interactions with CYP3A4 and P-gp inducers
Co-administration of apixaban with strong inducers of CYP3A4 and P-gp (such as rifampicin, phenytoin, phenobarbital or St. John's wort) can reduce the average apixaban exposure by 50%. Caution should be used when coadministered with CYP3A4 and P-gp inducers (see [Drug Interactions]
Interactions with other drugs affecting hemostasis:
When patients are also taking non-body anti-inflammatory drugs ( NSAIDS) including acetylsalicylic acid, apixaban should be taken with caution. In addition, combined use of apixaban with other platelet aggregation inhibitors or other anti-blood test drugs is not recommended (see [Substance Interactions]).
Spinal/epidural anesthesia or puncture:
For patients who receive antithrombotic drugs to prevent thrombosis, there is a risk of epidural or spinal cord hematoma complications when using spinal/epidural anesthesia or puncture, which may lead to long-term or water-lasting paralysis. The use of epidural indwelling catheters after surgery or the concomitant use of drugs that affect hemostasis may increase the risk of the above events. In addition, the first dose of apixaban must be taken at least 5 hours after the epidural or intrathecal catheter is removed. Trauma or repeated epidural or spinal puncture may also increase the above risk. Patients should be monitored intensively. Observe for signs and symptoms of neurological impairment (e.g., numbness or weakness in the legs, bowel or bladder dysfunction). If neurological impairment is observed, diagnosis and treatment must be initiated immediately, in patients who have already received anticoagulant therapy or who are preparing to receive anticoagulant therapy for the purpose of preventing thrombosis. Before performing spinal/epidural anesthesia or aspiration, the physician should weigh the potential benefits and risks.
There is no clinical experience in taking apixaban with an intrathecal or hard external indwelling catheter. If necessary, based on PK data, there should be an interval of 20 to 30 hours (i.e. 2 half-lives) between the last dose of apixaban and removal of the catheter. The drug should be stopped at least once before removal of the catheter, and apixaban can be taken at least 5 hours after removal of the catheter. Similar to all newer anticoagulants. Experience in patients receiving spinal/epidural anesthesia is limited; therefore, axaban should be administered with extreme caution to patients receiving spinal/epidural anesthesia.
髋骨骨折手术
目前尚无临床试验评价接受髋骨骨折手术患者服用阿哌沙班的有效性及安全性,因此,不推荐这些患者服用阿哌沙班。
Excipient information
This product contains lactose. Patients with rare hereditary galactose intolerance, Lapp lactase deficiency, or glucose-galactose malabsorption should not take this product.
Effects on the ability to drive and operate machinery
Apixaban has no effect on the ability to drive and operate machinery or the impact can be ignored.
[Drugs for pregnant and lactating women]
Pregnancy
Animal studies have not found direct or indirect reproductive toxicity of this product. There is currently no data on the use of apixaban in pregnant women, and apixaban is not recommended during pregnancy.
Breastfeeding women
It is not known whether apixaban or its metabolites pass into human milk. Existing animal experimental data show that apixaban can pass into breast milk. In rat milk, a high milk-to-maternal plasma drug concentration ratio was found (Cmax approximately 8, AUC approximately 30), possibly due to active drug transport into milk. Risks to newborns and infants cannot be excluded.
A decision must be made whether to discontinue breastfeeding or to discontinue/avoid apixaban treatment.
[Pediatric Use]
There is currently no data on the safety and effectiveness of apixaban in patients under 18 years of age.
[Drug for the Elderly]
No dose adjustment is required.
【药物相互作用】
CYP3A4及P-gp抑制剂:
当阿哌沙班与CYP3A4及P-gp强效抑制剂酮康唑(400mg,每日一次)合用时,阿哌沙班的平均AUC升高2倍,平均Cmax升高1.6倍,服用强效CYP3A4及P -gp抑制剂进行全身性治疗的患者不推荐服用阿哌沙班,此类抑制剂包括吡咯类抗真菌药(如酮康唑、伊曲康唑、伏立康唑及泊沙康唑)和HIV蛋白酶抑制剂(如利托那韦)(参见【注意事项】)
中度抑制阿哌沙班的消除途径CCYP3A4及/或P-gp)的活性物质可使阿哌沙班的血药浓度轻度升高。如地尔硫卓(360mg,每日一次),一种中度CYP3A4及弱P-gp抑制剂,可使阿哌沙班的平均AUC升髙1.4倍,平均Cmax升高1.3倍。 Naproxen (500 mg, single dose), a P-gp inhibitor that does not inhibit CYP3A4, increased the mean AUC of apixaban by 1.5-fold and the mean Cmax by 1.6-fold. No dose adjustment is required when apixaban is coadministered with non-potent CYP3A4 and/or P-gp inhibitors.
CYP3A4及P-gp诱导剂:
阿哌沙班与CYP3A4及P-gp强效诱导剂利福平合用时,可使阿哌沙班的平均AUC降低54%,平均Cmax降低42%。阿哌沙班与其它CYP3A4及P-gp强效诱导剂(如苯妥英、苯巴比妥或圣约翰草)合用时,也可能导致阿哌沙班的血药浓度降低。与上述药物合用时,无需调整剂量:但与一些强效CYP3A4及P-gp诱导剂合用时,应谨慎(参见【注意事项】)
抗凝药
在阿哌沙班(5mg,单次给药)与依诺肝素(40mg,单次给药)合用后,发现在抗Xa因子效应上有相加效应。
Caution should be exercised if the patient is taking any other anticoagulant drugs in combination due to an increased risk of bleeding (see Precautions).
Platelet aggregation inhibitors and nonsteroidal anti-inflammatory drugs:
No pharmacokinetic or pharmacodynamic interactions were observed when apixaban was coadministered with acetylsalicylic acid (325 mg once daily).
In phase I trials, when apixaban was combined with clopidogrel (75 mg, once daily), or combined with clopidogrel (75 mg, once daily) and acetylsalicylic acid (162 mg, once daily), compared with antiplatelet drugs alone, no corresponding increase in bleeding time, platelet aggregation, and coagulation parameters (PT, INR, APTT) was found.
Naproxen (500mg) is a P-gp inhibitor that can increase the average AUC of apixaban by 1.5 times and Cmax by 1.6 times, thereby prolonging the coagulation parameters of apixaban accordingly. When apixaban was combined with naproxen, no change in the effect of naproxen on arachidonic acid-induced platelet aggregation was found, and no clinically significant prolongation of bleeding time was observed.
Despite the support of the above data, individual patients may experience more significant pharmacodynamic responses when taking antiplatelet drugs and apixaban in combination. Caution should be exercised when coadministering apixaban with NSAIDs (including acetylsalicylic acid) as these drugs generally increase the risk of bleeding. In a clinical study of patients with acute coronary syndrome, triple therapy with apixaban, acetylsalicylic acid, and clopidogrel significantly increased the risk of bleeding. It is not recommended to use apixaban with drugs that can cause serious bleeding, such as unfractionated heparin and heparin derivatives (including low molecular weight heparin (LMWH)), oligosaccharides that inhibit coagulation factor Xa (such as fondaparinux sodium), thrombin II direct inhibitors (such as desirudin), thrombolytics, GPIIb/IIIa receptor antagonists, thienopyridines (such as clopidogrel), dipyridamole, dextran, sulfinpyrazone, vitamin K antagonists and other oral anticoagulants.
Other concomitant medications:
No clinically significant pharmacokinetic or pharmacodynamic interactions were observed when apixaban was coadministered with atenolol or famotidine. The coadministration of 10 mg apixaban and 100 mg atenolol did not have a clinically significant effect on the pharmacokinetics of apixaban. Compared with apixaban alone, the average AUC and Cmax of apixaban were reduced by 15% and 18%, respectively. Coadministration of 10 mg apixaban and 40 mg famotidine had no effect on the AUC or Cmax of apixaban.
The impact of apixaban on other drugs:
In vitro experiments found that apixaban did not inhibit the activity of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2D6 or CYP3A4 when the concentration was far beyond the peak plasma concentration in patients (I C50>45μM), has a weak inhibitory effect on CYP2C19 activity (IC50>20μM). When the concentration of apixaban is as high as 20μM, it does not induce CYP1A2, CYP2B6, CYP3A4/5. Therefore, apixaban is not expected to change the metabolic elimination rate of concomitant drugs metabolized by these enzymes. Apixaban is not a significant P-gp inhibitor. As discussed below, apixaban did not have clinically meaningful effects on the pharmacokinetics of digoxin, naproxen, or atenolol in trials with healthy volunteers.
Digoxin: Taking apixaban (20 mg, once daily) and the P-gp substrate digoxin (0.25 mg, once daily) at the same time may have no effect on the AUC of digoxin. Therefore, apixaban does not inhibit P-gp-mediated substrate transport.
Naproxen: The simultaneous administration of a single dose of apixaban (10 mg) and a commonly used non-steroidal anti-inflammatory drug naproxen (500 mg) has no effect on the AUC of naproxen.
Atenolol: The simultaneous administration of a single dose of apixaban (10 mg) and a commonly used β-blocker atenolol (100 mg) does not change the pharmacokinetics of atenolol.
[Drug overdose]
There are no antidotes for apixaban. Apixaban overdose may result in an increased risk of bleeding. When bleeding complications occur, the drug should be stopped immediately and the cause of bleeding should be identified. Appropriate treatment measures should be considered, such as surgical hemostasis, transfusion of fresh frozen plasma, etc.
In a controlled clinical trial, healthy volunteers received up to 50 mg of apixaban orally for 3 to 7 days (25 mg twice daily for 7 days or 50 mg once daily for 3 days) [equivalent to 10 times the maximum recommended daily human dose] without clinically significant adverse reactions.
A preclinical trial in dogs found that oral administration of activated charcoal within 3 hours of apixaban administration reduced apixaban exposure; therefore, the use of activated charcoal may be considered in the management of apixaban overdose.
If life-threatening bleeding cannot be controlled with the above treatments, recombinant factor VIIa may be considered. However, there is currently no experience with recombinant factor VIIa in patients taking apixaban. Repeated administration of recombinant factor VIIa may be considered, and the dose may be adjusted based on improvement in bleeding.
[Pharmacology and Toxicology]
Pharmacological effects
Apixaban is a potent, orally effective, reversible, direct, highly selective factor Xa active site inhibitor, and its antithrombotic activity does not depend on antithrombin III. Apixaban inhibits free and thrombus-bound factor Xa and inhibits prothrombin activity. Apixaban has no direct effect on platelet aggregation, but indirectly inhibits thrombin-induced platelet aggregation. By inhibiting factor Xa, apixaban inhibits thrombin generation and inhibits thrombosis. Preclinical trial results in animal models show that apixaban has antithrombotic effects and can prevent arterial and venous thrombosis at a dose level that does not affect hemostatic function.
The pharmacodynamic effect of apixaban is reflected in its mechanism of action (inhibition of factor At the expected therapeutic dose, the change range of these coagulation parameters is very small and the variability is large. It is not recommended to use these parameters to evaluate the pharmacodynamic effect of apixaban.
In in vitro studies using a variety of commercially available anti-factor Xa kits, it can be seen that axaban reduces the enzymatic activity of factor Within a wide dose range, there is a linear relationship between the concentration of apixaban and its anti-factor Xa activity. The accuracy of the Rotachrom test meets the requirements of clinical laboratories. After taking apixaban, the changes in anti-factor Xa activity caused by changes in dose and concentration of apixaban are more significant and smaller than the changes in coagulation parameters.
After taking 2.5 mg of asaban twice daily, the steady-state peak and trough values predicting its anti-factor XA activity are 1.3 IU/ml (5th/95th percentile: 0.67-2.4 IU/ml) and 0.84 IU/ml (5th/95th percentile: 0.37-18) respectively. IU/ml), that is, the peak/trough ratio of anti-factor Xa activity during the dosing interval is less than 1.6 times.
Although exposure should not be routinely monitored while taking apixaban, the Rotachrom Anti-Xa Factor Assay may be useful in special circumstances where knowledge of apixaban exposure is needed to aid clinical decision-making, such as overdose and emergency surgery.
Toxicological studies
Genetic toxicity: The results of apixaban Ames test, Chinese hamster ovary cell chromosome aberration test, and rat bone marrow micronucleus test were all negative.
Reproductive toxicity: The results of rat fertility and early embryonic development toxicity tests show that when apixaban is administered at a dose of 600 mg/kg, maternal toxicity can be seen on coagulation parameter values, but no obvious impact on maternal fertility and no obvious impact on the growth and development of offspring. Pregnant rats and pregnant rabbits were administered apixaband 30 times orally. 00mg/kg/day and 1500mg/kg/day, no drug-related obvious abnormalities in the growth and development of the offspring were found; the results of the perinatal reproductive toxicity test in rats showed that the NOAEL for the impact on maternal reproductive function was 1000mg/kg/day, and the NOAEL for the impact on the growth and development of the offspring was less than 25mg/kg/day.
Carcinogenicity: A 104-week carcinogenicity test was conducted on mice and rats after oral administration of apixaban. Male and female mice were administered doses of 1,500 mg/kg/day and 3,000 mg/kg/day respectively. No dose-related increase in tumor incidence was found. Rats were orally administered apixaban at doses up to 600 mg/kg/day, and no drug-related increase in tumor incidence was found.
[Pharmacokinetics]
Absorption
In the 10 mg dose range, the absolute bioavailability of apixaban is approximately 50%. Apixaban is rapidly absorbed and reaches its maximum concentration (Cmax) 3 to 4 hours after taking it. Food has no effect on the AUC or Cmax of apixaban 10 mg. Apixaban can be taken with or without meals.
In the 10 mg dose range, apixaban exhibits linear pharmacokinetic characteristics and is dose-dependent. When the dose of apixaban is ≥25 mg, it shows dissolution-limited absorption and decreased bioavailability. Apixaban's exposure parameters exhibit low to moderate variability, with intra-individual coefficients of variation (CV) of approximately 20% and inter-individual coefficients of approximately 30%.
Distribution
In the human body, the binding rate to plasma proteins is approximately 87%. The volume of distribution (Vss) is approximately 21 liters.
Metabolism
The main site of biotransformation of apixaban is o-demethylation or hydroxylation of the 3-piperidinone group. Apixaban is mainly metabolized by CYP3A4/5, and to a lesser extent by CYP1A2, 2C8, 2C9, 2C19 and 2J2. Prototype apixaban is the major drug-related component in human plasma and no active circulating metabolites have been identified. Apixaban is a substrate of the transporter P-gp and breast cancer resistance protein (BCRP).
Excretion
Apixaban can be eliminated through a variety of pathways. After the human body is given apixaban, about 25% appears as metabolites, and the vast majority are detected in feces. Renal excretion accounts for approximately 27% of total clearance. Additionally, additional biliary excretion has been found in clinical trials and additional direct intestinal excretion has been found in nonclinical trials. The total clearance rate of apixaban is approximately 3.3L/h, and its half-life is approximately less than 12 hours.
Special Populations
Renal Impairment
Renal impairment has no effect on the maximum plasma concentration of apixaban. Apixaban exposure increases with decreases in renal function (as assessed by creatinine clearance). Compared with those with normal creatinine clearance, the area under the plasma concentration curve (AUC) of apixaban in patients with mild renal impairment (creatinine clearance 51-80ml/mim), moderate impairment (creatinine clearance 30-50ml/mim) and severe impairment (creatinine clearance 15-29ml/mim) increased by 16%, 29% and 44% respectively. Renal impairment has no significant effect on the relationship between apixaban plasma concentration and anti-FXa activity.
Liver damage
In a study comparing patients with mild liver damage (Child Pugh class A, including 6 patients with a score of 5 and 2 with a score of 6) and moderate liver damage (Child Pugh Level B, including 6 cases with a score of 7 and 2 cases with a score of 8) and healthy subjects (16 cases), after single administration of 5 mg of apixaban, there was no change in the pharmacokinetics and pharmacodynamics of apixaban in patients with liver damage. The changes in anti-FXa activity and INR in patients with mild or moderate liver damage were comparable to those in healthy subjects.
Elderly patients
The plasma concentration of elderly patients (older than 65 years old) is higher than that of younger patients, and the average AUC is approximately 32% higher.
Gender
Women’s exposure to apixaban is approximately 18% higher than that of men, and no dose adjustment is required.
Race and ethnicity
Results from a phase I clinical trial showed no significant differences in the pharmacokinetics of apixaban between Caucasians/Caucasians, Asians, and blacks/African Americans. A population pharmacokinetic analysis of apixaban in patients undergoing elective hip or knee replacement surgery was consistent with the results of the phase I trial described above.
Weight
Compared with patients weighing 65kg to 85kg, apixaban exposure in patients weighing >120kg was approximately 30% lower, and in patients weighing <50kg, apixaban exposure was increased by approximately 30%, without dose adjustment.
Pharmacokinetic/pharmacodynamic relationship
The pharmacokinetic/pharmacodynamic (PK/PD) relationship between apixaban plasma concentration and several pharmacodynamic endpoints (anti-factor The relationship between apixaban concentration and factor Xa activity best fit a linear model. The PK/PD relationship in patients undergoing elective hip or knee replacement surgery was consistent with the results in healthy subjects.
[Storage] Store below 30℃.