Aficamten-MYQORZO manual Chinese introduction
1. Common name: aficamten tablets, aficamten
Product name: Xingshuping,MYQORZO
2. Who can take Afkaita tablets? Indications
aficamten (aficamten)-MYQORZO is indicated for the treatment of adults with symptomatic (New York Heart Association, NYHA, Class II-III) obstructivehypertrophic cardiomyopathy (oHCM) to improve functional capacity and symptoms.
3. What are the side effects of Afkaita tablets?
Hypertension was the only adverse effect in clinical studies of Afkatai. Afkatai comes with a box warning about the risk of heart failure. Afkatecan reduce left ventricular ejection fraction (LVEF) and may lead to heart failure due to systolic dysfunction.
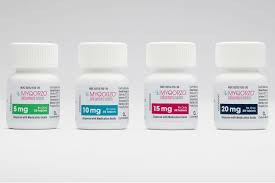
4. How to take Afkaita tablets
1. Evaluation before and during the use of Afkatide: It is not recommended to start use or increase the dose in patients with left ventricular ejection fraction (LVEF) <55%. Patients may develop heart failure while taking Afkate. Periodic assessment of LVEF and Valsalva left ventricular outflow tract gradient (LVOT-G) is required to achieve appropriate target Valsalva LVOT-G while maintaining LVEF ≥50% and avoiding symptoms of heart failure.
2. Recommended dose: The starting dose of Afkatai is5 mg, taken orally once a day. Increase the dose by 5 mg every 2 to 8 weeks until the maintenance dose or the maximum recommended dose of 20 mg once daily is reached. The maintenance dose of Afkatide is individualized based on the patient's LVEF and LVOT-G.
3. How to use: Afkatai should be taken once a day, with or without food at approximately the same time every day. Swallow the tablet whole.
4. Missed dose: If you miss a dose, take it as soon as possible on the same day. The next scheduled dose should be taken at your normal time the next day. Two doses of Afkat should not be taken on the same day.
5. How to provide and store Afkaita tablets
Aficamten tablets are available as purple film-coated tablets containing5mg, 10mg, 15mg or 20mg aficamten. Store quickly at 20°C to 25°C (68°F to 77°F); tolerances allowed between 15°C to 30°C (59°F to 86°F).
6. How Afkaitai works
Afkatide is an allosteric and reversible inhibitor of cardiac myosin motor activity. Afkatide reduces the force generated by myosin at the cardiomyome, which contributes to the pathophysiology of HCM. In patients with HCM, myosin inhibition with Afkatide reduces myocardial contractility and left ventricular outflow tract (LVOT) obstruction.
7. What will happen if you take too much Afkatai?
There have been no reports of overdose with Afkatai. Cardiovascular effects of overdose may include decreased LVEF, heart failure, and hypotension. Signs and symptoms of heart failure, such as dyspnea, fatigue, leg edema, or elevated NT-proBNP, should prompt evaluation of cardiac function. Discontinue treatment with Afkat. Provide medical support measures to maintain hemodynamic stability and monitor left ventricular function.
8. Who cannot use Afkaita tablets?
It is forbidden to use Afkaeta tablets with rifampicin at the same time.
9. When will Afkaita tablets be released?
1. The U.S. Food and Drug Administration (FDA) approved it on December 19, 2025, with the trade name Myqorzo;
2. The European Medicines Agency (EMA) granted Myqorzo marketing authorization on December 12, 2025;
3. On December 17, 2025, the State Food and Drug Administration approved the listing of Afkaitai Tablets (trade name: Xingshuping).
References:https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fd778507-1274-4d1a-a659-5431d55c543a
[ 免责声明 ] 本页面内容来自公开渠道(如FDA官网、Drugs官网、原研药厂官网等),仅供持有医疗专业资质的人员用于医学药学研究参考,不构成任何治疗建议或药品推荐。所涉药品可能未在中国大陆获批上市,不适用于中国境内销售和使用。如需治疗,请咨询正规医疗机构。本站不提供药品销售或代购服务。
.jpeg)


















