Storage conditions and precautions for Teritusumab/Telivac
Teritusumab (Teclistamab-cqyv) is an innovative immunotherapy drug. As a bispecific antibody, it can simultaneously target B cell maturation antigen (BCMA) and T cell receptor (CD3) activate the patient's immune system to attack cancer cells and are mainly used to treat patients with relapsed or refractory multiple myeloma (MM). Due to its unique mechanism of action, teritusumab has achieved remarkable efficacy in cancer treatment, especially in the treatment of multiple myeloma. In order to ensure its effectiveness and safety in treatment, the storage conditions and usage precautions of drugs are very important.
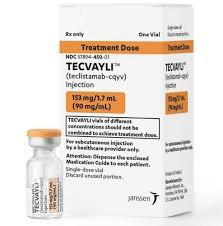
Territuzumab is a biopharmaceutical that requires refrigerated storage, and its storage conditions are very strict. According to the drug package insert, teritusumab should be stored in a refrigerated environment at 2°C to 8°C. This temperature range can ensure the stability and effectiveness of the drug and prevent it from failing in high temperature or extremely low temperature environments. It is important to note that teritusumab should not be frozen. Freezing can cause the drug's structure to change, thereby losing its therapeutic effect.
Medicines should be stored in their original packaging, which not only helps prevent the medicines from coming into contact with the outside world and the effects of light and moisture, but also reduces the risk of contamination of the medicines. Sunlight has a certain damaging effect on many biopharmaceuticals, so direct exposure to light should be avoided during storage.
Teritusumab should be stored out of the reach of children to avoid ingestion or misuse. For patients with children at home, special attention needs to be paid to the storage location of medicines. The medicines should be stored in locked cabinets as much as possible to ensure that children cannot easily access them. In addition, it is also necessary to ensure that the medicine is not affected by excessive vibration or high temperature during transportation. It is best to use cold chain transportation during transportation to ensure that the medicine reaches the destination stably at a suitable temperature.
Keyword tags: teritusumab, Teclistamab, storage conditions, refrigerated storage, drug use, side effects, immunotherapy, drug management, myeloma treatment
Reference materials:https://go.drugbank.com/drugs/DB16655
[ 免责声明 ] 本页面内容来自公开渠道(如FDA官网、Drugs官网、原研药厂官网等),仅供持有医疗专业资质的人员用于医学药学研究参考,不构成任何治疗建议或药品推荐。所涉药品可能未在中国大陆获批上市,不适用于中国境内销售和使用。如需治疗,请咨询正规医疗机构。本站不提供药品销售或代购服务。
.jpeg)


















