The price of Dabrafenib has "transformed" from tens of thousands of yuan to several thousand yuan. How can it benefit patients with BRAF mutations?
With the deepening of the concept of precision medicine, tumor treatment is undergoing a profound change - "What we treat is no longer lung cancer or melanoma, but BRAF V600 mutations." In this context, the BRAF inhibitor dabrafenib (Dabrafenib) has become increasingly prominent. It is not only effective as a single drug, but also forms a "dual-target combination" regimen with the MEK inhibitor trametinib (Trametinib), becoming an efficient standard treatment in the fields of non-small cell lung cancer and melanoma. Now, with the drug being launched in China and included in the national medical insurance, the "exorbitantly expensive drug" that once cost hundreds of thousands of yuan per year has become affordable. At the same time, high-quality generic drugs approved in Laos and other regions have further built a diversified "life defense line" for patients. This article will provide an in-depth analysis of the therapeutic value, price changes and standardized medication routes of dabrafenib.
Dabrafenib: How to accurately “lock” BRAF mutation and rewrite the patient survival curve?
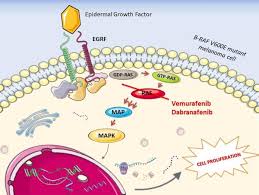
Dabrafenib (trade name: Tafinlar) is an oral, potent, selective BRAF inhibitor developed by GlaxoSmithKline (GSK). It mainly targets the BRAF V600E mutation - this mutation is like a "runaway switch" that continuously activates the MAPK signaling pathway, leading to crazy proliferation and metastasis of tumor cells.
Different from the "indiscriminate attack" of traditional chemotherapy, dabrafenib's effect is extremely "discerning": it precisely binds to the mutated BRAF protein, blocks abnormal signaling, thereby inhibiting tumor growth and inducing apoptosis. First approved for BRAF since2013 Since V600mutation-positive melanoma, its indications have been expanded to non-small cell lung cancer, thyroid cancer and solid tumors. Especially when combined with trametinib, it can significantly delay drug resistance and improve efficacy. Median progression-free survival (PFS) and overall survival (OS) data have repeatedly refreshed clinical records.
Domestic accessibility revolution: The leap from “exorbitantly priced drugs” to “medically insured drugs”
Once upon a time, the annual treatment cost of dabrafenib was prohibitive for most families. The turning point occurred when the drug was included in the National Medical Insurance List. This policy change directly promoted the transformation of anti-cancer treatment from a "luxury" to a "necessity".
Up to now, dabrafenib has been widely available in China, and its common specifications are:
75mg × 120tablets/box, the latest domestic market price is about eight to nine thousand yuan. After being reimbursed by medical insurance, the patient's out-of-pocket payment ratio is significantly reduced. The specific amount needs to be based on local medical insurance policies (usually the reimbursement ratio ranges from 50% to 80%). It is recommended that patients consult the local hospital pharmacy or medical insurance department to obtain accurate information.
Calculated based on conventional treatment doses, with medical insurance coverage, the patient's annual out-of-pocket expenses can be reduced from hundreds of thousands of yuan to less than tens of thousands of yuan. This is not only a reduction of economic burden, but also a substantial support for life hope.
Overseas generic drug options: Laos version of dabrafenib, building"second lifeline"
For some patients with limited medical insurance reimbursement rates or who wish to further reduce long-term treatment costs, the generic version of dabrafenib, officially approved by the Lao Ministry of Health, provides a proven alternative.
Lao generic drugs: The drug ingredients are basically the same as the original drugs and are prepared under standard production conditions. The current market price is about 2,000 RMB/ box (75mg*120 capsules specifications), and the price advantage is obvious.
IMPORTANT NOTE:
1.Quality first: When choosing generic drugs, be sure to verify whether the pharmaceutical factory has been approved by the Lao Ministry of Health and whether the product has a complete quality inspection report.
2.Formal channels: Purchase through formal platforms or pharmacies with cross-border drug sales qualifications and avoid personal channels to prevent counterfeit and inferior drugs.
3.Doctor-patient communication: Be sure to fully communicate with the attending doctor before taking medication to ensure that the overseas drug purchase plan is consistent with the overall treatment strategy.

In-depth analysis: PriceThe logic of medical accessibility behind the “dive”
Dabrafenib’s path from “sky-high price” to “people-friendly” reflects the three major engines for improving global drug accessibility:
1.National medical insurance negotiation “Volume for price”: China’s medical insurance system has significantly lowered the entry price of innovative drugs through collective negotiations, allowing pharmaceutical companies to obtain stable market expectations while allowing patients to share in the results.
2.The "catfish effect" of generic drugs: Although legal generic drugs approved in Laos and other regions have not directly entered the Chinese market, their existence has formed a price reference system and indirectly promoted the rationalization of the global drug pricing system.
3.Evolution of treatment models: Combination regimens such as "dabrafenib+trametinib" have become standard, improving treatment efficiency, reducing indirect costs caused by ineffective treatment, and improving the "value for money" of drugs.
Who does dabrafenib fight for? Beneficiary groups under accurate portrait
According to the latest clinical guidelines and practices, the following groups are the main beneficiaries of dabrafenib (single agent or combination):
1.Patients with unresectable or metastatic melanoma confirmed to be BRAF V600E mutation-positive by genetic testing.
2.BRAF V600EMutation-positive patients with advanced non-small cell lung cancer (combination with trametinib has become the first-line standard).
3.BRAF V600Patients with locally advanced or metastatic anaplastic thyroid cancer who are mutation-positive.
4.Patients with various solid tumors who have failed previous treatments and carry BRAF V600 mutations (refer to the latest clinical trial data).
5.Patients who pursue high-quality long-term survival, hope to delay chemotherapy intervention, and maintain a better living condition.
6.The core first step: genetic testing. BRAF V600 mutation testing via tumor tissue or liquid biopsy is an indispensable "navigator" before considering the use of any targeted drugs.
Meeting the Challenge: Understanding and Managing Common Side Effects of Dabrafenib
The efficacy of dabrafenib is clear, but like most targeted drugs, it may be accompanied by a series of side effects. Common ones include:
1.Fever: The incidence is high and may be related to the release of cytokines. It can usually be controlled through symptomatic treatment and dose adjustment.
2.Skin reactions: including rash, increased photosensitivity, hyperkeratosis, etc. Pay attention to sun protection and skin care.
3.Joint pain and fatigue: affect the quality of life, but most are mild to moderate.
4.Rarely common side effects that require vigilance: such as bleeding, arrhythmia, new primary skin malignant tumors, etc.
The key to management lies in "monitoring and communication": patients need to regularly review blood routine, liver and kidney function, electrocardiogram, etc., pay close attention to any abnormal signals in the body, and provide timely feedback to the medical team. Your doctor may manage side effects by adjusting the dose, suspending the medication, or providing symptomatic and supportive care. Do not stop the medication on your own.
Conclusion: In the era of precision medicine, seize your life
The story of dabrafenib is a story about scientific breakthroughs, policy benefits and patient empowerment. It symbolizes that the anti-cancer war has entered a new stage of "precision guidance" from"fuzzy bombing". Nowadays, the door to medical insurance has been opened, and legal generic drug channels have also provided supplements for multi-level needs. For patients with BRAF V600 mutations, the key is to take the initiative to undergo genetic testing, discuss treatment plans with doctors, and obtain drugs from regular channels.
In the game of life, information is power and choice is direction. When the most advanced treatments become within reach, hope is no longer an unreachable star, but a light that can be held in your hands.
xa0
References:
1.United StatesFDADrug insert (dabrafenib): https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/202806s020lbl.pdf
2.China National Medical Products Administration (NMPA) drug query platform: http://www.nmpa.gov.cn/WS04/CL2042/
3.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1492
4.GlaxoSmithKline (GSK) official product information: https://www.gsk.com/en-gb/products/tafinlar/
[ 免责声明 ] 本页面内容来自公开渠道(如FDA官网、Drugs官网、原研药厂官网等),仅供持有医疗专业资质的人员用于医学药学研究参考,不构成任何治疗建议或药品推荐。所涉药品可能未在中国大陆获批上市,不适用于中国境内销售和使用。如需治疗,请咨询正规医疗机构。本站不提供药品销售或代购服务。
.jpeg)


















