Comparative analysis of different versions of Anamorelin medicine
Anamorelin(Anamorelin) is a selective ghrelin receptor agonist, mainly used for the symptomatic treatment of cancer anorexia-cachexia syndrome (CACS). At present, the drug has not been approved for marketing in my country. If it is needed for clinical use, patients usually need to obtain it through overseas channels. Therefore, the differences in versions between different countries and manufacturers have become a key concern for patients and their families when making actual choices.
Judging from the market situation, the original version of Anamorelin mainly comes from Japan and is produced by pharmaceutical companies with mature R&D systems. It is the version that has been used internationally for a longer time and has relatively sufficient research data. The common specifications of the Japanese version of Anamorelin are50 mg × 100 tablets. The dosage design is consistent with overseas studies and instructions, and it has high reliability in terms of drug stability, batch consistency and quality control. In terms of price, the original version is usually sold in overseas pharmacies at around 4,000 yuan, which will fluctuate depending on the exchange rate and purchasing channels.
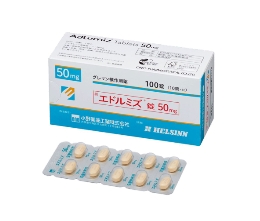
At the same time, some countries have begun to produce generic versions of anamorelin, among which the anamorelin produced by Lucius Pharmaceutical Factory in Laos has attracted more attention. From the perspective of ingredients, the core active ingredients of this generic drug are consistent with the original drug. Anamorelin is also used as the main drug base, and there is no essential difference in the theoretical mechanism of action. Its specification design is also basically based on the original product, making it easier for patients to use it according to the established dosage plan. Compared with the original version, generic drugs have more advantages in terms of price. Each box is usually priced at about more than 2,000 yuan, providing a more affordable option for long-term medication patients.
What needs to be viewed rationally is that the difference between original drugs and generic drugs is not simply reflected in price. Although generic drugs are highly consistent in terms of active ingredients, there may still be certain gaps with original drugs in aspects such as excipient selection, production technology, and quality monitoring systems. For patients with greater financial pressure, choosing generic drugs from reliable sources after evaluation by doctors is also a realistic and feasible solution.
Reference materials:https://pmc.ncbi.nlm.nih.gov/articles/PMC4677053/
[ 免责声明 ] 本页面内容来自公开渠道(如FDA官网、Drugs官网、原研药厂官网等),仅供持有医疗专业资质的人员用于医学药学研究参考,不构成任何治疗建议或药品推荐。所涉药品可能未在中国大陆获批上市,不适用于中国境内销售和使用。如需治疗,请咨询正规医疗机构。本站不提供药品销售或代购服务。
.jpeg)


















